Stem Cell Biology
A major research focus of our laboratory is the biology of the neural crest, a transient embryonic stem cell population central to the evolution of vertebrates and endowed with one of the broadest developmental potentials in vivo. It is assumed that a third of all congenital birth defects result from failures in neural crest stem cell (NCSC) development, illustrating the significance of this cell population. Moreover, there is increasing evidence that an embryonic NCSC gene expression signature is re-activated during melanoma initiation, metastasis formation, and therapy resistance, suggesting a functional involvement of a NCSC program in tumors originating from neural crest derivatives. Likewise, we have recently shown that neural crest-derived peripheral glia in the skin adopt NCSC-like features upon injury to support wound healing. Thus, it is highly relevant to study the biology of NCSCs and to characterize "NCSC states" in development, disease, and regeneration.
Neural crest stem cells in development
Along with fate-restricted cells, the neural crest harbors many cells termed neural crest stem cells (NCSCs) that display self-renewal capacity and multipotency. Using inducible and conditional tissue-specific gene ablation in mice, in conjunction with functional analyses in primary cell cultures and, more recently, in cultures derived from human embryonic stem cells, we have been characterizing molecular mechanisms regulating self-renewal of NCSCs and promoting NCSC fate decisions and differentiation. In addition, we have performed genetic single cell fate mapping in mice to demonstrate multipotency of premigratory and migratory neural crest cells in vivo, thus resolving a long-standing controversy in the field about the in vivo potential of neural crest cells. We are utilising state-of-the-art multi-omics technologies, including single cell RNA sequencing, single cell ATAC sequencing, and translatome analyses, to discover and characterize the cellular states and mechanisms underlying multilineage differentiation of NCSCs and disease formation.
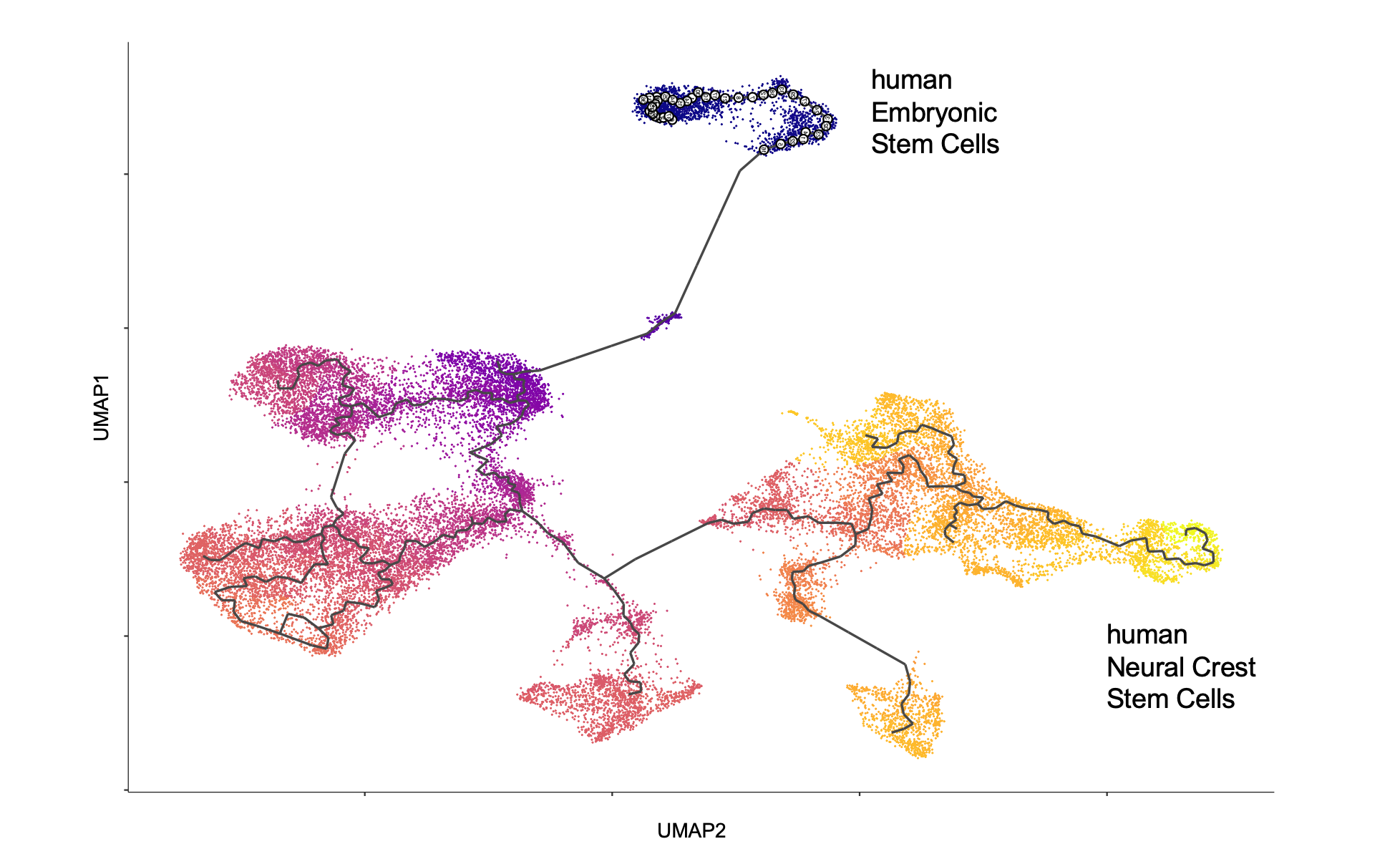
- Pseudotime trajectory inferred from single-cell RNA sequencing unveils development of neural crest stem cells from human embryonic stem cells.
NCSC-like cells in tissue regeneration
Apart from studying the biology of NCSCs during embryonic development, we are investigating ‘stemness’ properties of cells present in adult neural crest-derived structures, such as the melanocyte compartment and peripheral nerves. Intriguingly, we were able to isolate cells very similar to NCSCs from adult murine and human skin. Using lineage tracing and gene depletion technologies, we have shown that such NCSC-like cells emerge by injury-induced reprogramming of peripheral nerve cells in vivo and support skin wound healing by secretion of a plethora of tissue repair factors associated with inflammation, angiogenesis, ECM remodeling, and fibrosis, and other processes previously implicated in wound healing and cancer. Characterization of NCSC-like cells and their influence on other skin cell types at the single cell level will help us to better understand how neural crest-derived cells interact with surrounding cells to support tissue repair and regeneration.
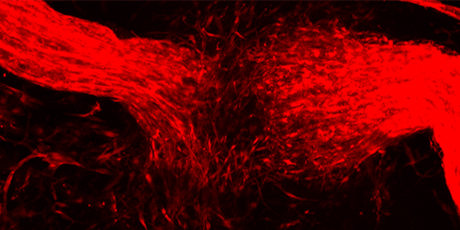
- Skin injury induces NCSC-like glial cells to disseminate from peripheral nerves into the wound bed.
NCSC-like cells in tumorigenesis
The identification of adult NCSC-like cells in the skin prompted us to address their potential implication in disease, notably in the formation of melanoma, an aggressive skin tumor originating from melanocytes. Using human melanoma cells and genetic mouse models of melanoma, together with multi-omics technologies, we are addressing the functional implication of NCSC features in melanoma initiation, growth, and metastasis formation. In particular, we have revealed NCSC transcription factors, growth factor signaling pathways, and metabolic programs to control melanoma progression. We have also demonstrated a central role of the epigenetic regulator Ezh2 in promoting tumor formation and in modulating tumor-immune cell interactions, pointing to novel treatment strategies. Our aim is to obtain a comprehensive picture of how NCSC-like states contribute to tumor progression in mouse models and human patients.
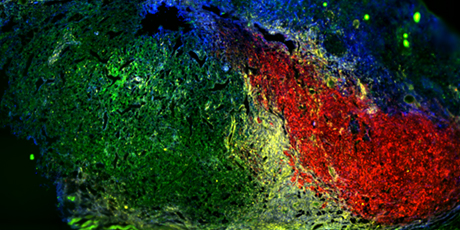
- Tumor formation from multiple cellular clones in a genetically engineered melanoma mouse model.
Our research is performed by an international and highly interdisciplinary team. By assuming a bridging function between basic and clinical research, our lab has been collaborating successfully with other scientists and, in particular, many clinicians to investigate parallels between embryonic development and disease.The study of normal stem cell development thus continues to provide important insights into mechanisms implicated in tissue regeneration and tumorigenesis. For a synopsis and goals of our research, see the video below.
Video by Johanna Diener